The outbreak of new coronary pneumonia led to a surge in demand for masks. At present, the domestic epidemic situation is tending to ease, the number of infected people in Europe, Italy, Japan, South Korea and other countries continues to rise, and Iran has attracted global attention with a very high lethal rate. In view of this, the business opportunities of masks quickly moved abroad, and the export of masks has become a hot business in the epidemic.
American mask standards and certification requirements
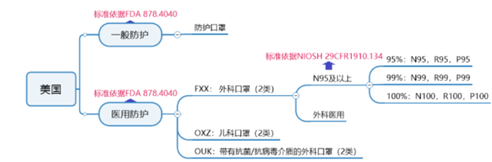
U.S. general protective mask certification requirements:
According to the US FDA medical certification, the process is:
① Fill in the application form and confirm the information;
② ②Get the PIN code and pay the annual fee;
③ Issue the registration number;
④Product export.
American medical surgical mask certification requirements:
According to the US FDA medical class II certification, the process is:
①Product testing (performance testing, biological testing);
② Prepare 510K documents and submit to FDA for review;
③FDA issued 510K approval letter; ④Complete factory registration and machine listing;
⑤ Product export.
U.S. medical N95 and above 9 mask certification requirements:
According to the NIOSH certification standard, companies need to send samples to the NIOSH laboratory for testing, and submit technical information (including quality system part information) to the NIOSH document review. After the document review and test are passed, NIOSH approves and issues the approval
EU mask standards and certification requirements
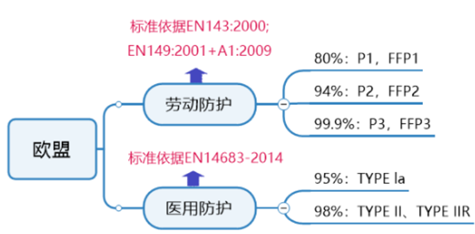
EU general protective mask certification requirements:
The European standards for personal protective masks are EN149 and EN143. Protective masks need to meet the requirements of the European Union's Personal Protective Equipment Directive (PPE), audit the enterprise quality management system and CE technical documentation, and obtain the CE certificate of the PPE regulations after passing the audit.
EU medical protective mask certification requirements:
The corresponding European standard for medical masks is EN14683. According to the requirements of the medical device regulations 2017/745/EU, mask products can be managed according to a class of devices. Depending on whether the product is provided in a sterile or non-sterile state, the certification model differs.
Australian & New Zealand mask standards and certification requirements
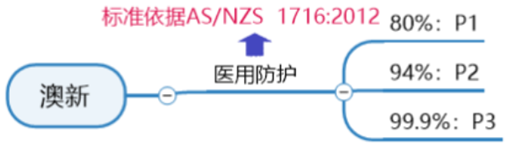
Requirements for certification of ANZ Medical Protective Mask:
AS/NZS1716: 2012 is the standard for respiratory protection devices in Australia and New Zealand. The standard specifies the procedures and materials that must be used in the manufacturing process of anti-particle masks, as well as the determined tests and performance results to ensure their safe use.
Korean mask standards and certification requirements
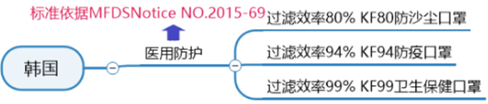
Korean medical mask certification standards:
The Korean mask standard KF (Korean filter) series is the mainstream mask standard issued by the Ministry of Food and Drug Safety (MFDS) in Korea
(Regulations on the Approval, Notification, and Evaluation of Quasi-Drugs).
Japanese mask standards and certification requirements
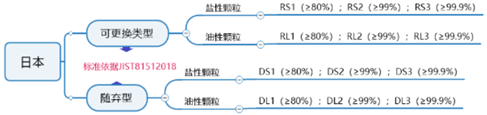
The Japanese JIST81512018 standard is a standard for respiratory protection devices, and is also a verification standard of the Ministry of Health, Labor and Welfare (MHLW) of Japan. This certification is required for export to Japan.
Our company recognizes that Unitech's testing organization can apply for mask testing certification for mask customers in the United States and Europe. If you have related products that require certification testing, you are welcome to call our staff directly at 400-880-1556 to obtain detailed fees. Quotation and cycle information. China Unicom has rich experience and successful cases in testing and certification, worthy of your trust and support!