introduction
Recently, since the three ministries have clearly required that all exported medical supplies must have a medical device product registration certificate approved by the drug regulatory authority in order to be released, many manufacturers who initially wished to obtain CE certification for medical masks through self-declaration began to export to Europe. Attention turned to protective masks. Protective masks in the EU are personal protective equipment PPE, which must meet the requirements of Regulation (EU) 2016/425, and perform performance tests in accordance with EN 149:2001 + A1:2009.
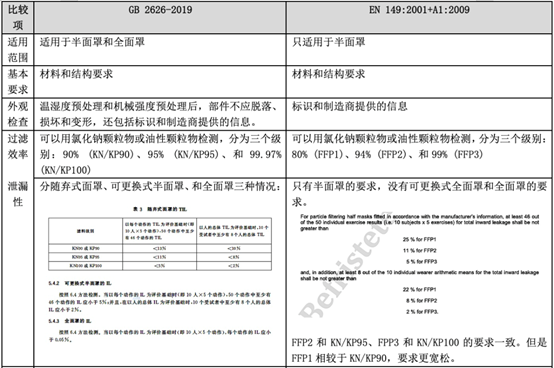
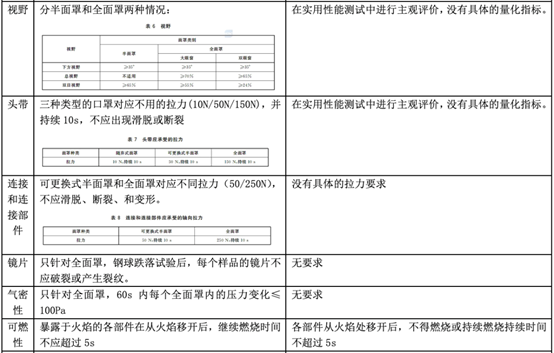
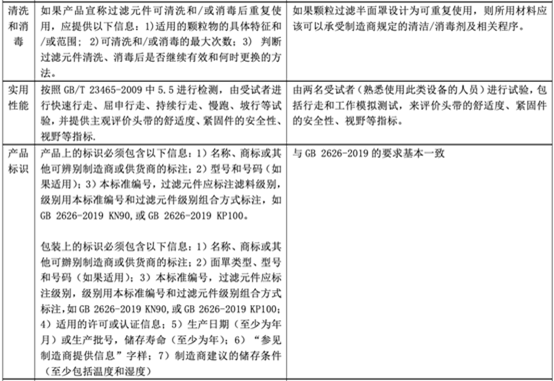
In fact, regarding personal protective masks, there is a corresponding standard GB 2626-2019 in China, but there is no equivalent conversion relationship with EN 149, so what is the difference between the two? The following table will explain in detail the application scope, basic requirements, and testing items of the standard.
Comparison between GB 2626-2019 and EN 149:2001 + A1:2009


to sum up
As can be seen from the above table, the EU personal protective mask test standard EN 149:2001 + A1:2009 and the domestic standard GB 2626-2019 have certain similarities in test items and index requirements, such as dead space, flammability, clean and Disinfection, practical performance and product identification, etc.
However, since GB 2626 is applicable to three types of masks, the overall inspection items will exceed EN149. In some indicators (such as: particle filtration efficiency), the requirements of GB 2626 are even higher than EN 149 (for FFP1). Therefore, it is a good strategy for manufacturers to perform performance testing in accordance with GB 2626 before submitting to EN 149 for inspection.